What is a Patent?
A patent is an exclusive privilege given to the author by the State to prohibit anyone from utilizing, creating, and selling an invention for a specified duration of time. It applies to a monopoly right on an invention. However, not all inventions are patentable and nor is it essential that inventions be protected solely by patent. Other forms of intellectual property rights can protect the final product which results from an invention. The primary objective for enacting patent law is to encourage inventors to make a greater contribution to their field by granting them exclusive rights to their inventions. In India, an innovation referring to a new product or procedure that involves the inventive phase and is capable of industrial use can be patented. Nonetheless, this does not, therefore, come under the scope of innovations which are non-patentable as provided for in sections 3 and 4 of the (Indian) Patents Act 1970. A patent application can be filed, either alone or jointly, by true and first inventor or his assignee.
The Patents act, 1970
The history of patent law in India begins with the enactment of the Indian Patents and Designs Act in 1911. Subsequently, in 1972, the current Patents Act 1970, came into effect, amending and consolidating the established patent legislation in India. The Patent Act essentially is based on the Justice Ann report ‘s recommendations, an Ayyangar Committee led by Iyengar Rajagopala. One of the recommendations was the granting of process patents in relation to drug, drug, food, and chemical inventions. The Patents Act, 1970 was amended once again by the Patents (Amendment) Act, 2005 concerning the extension of product patents in all areas of technology including food, medicine, chemicals, and microorganisms.
Patents act is the subset of the Intellectual property laws and a branch which deals with new inventions. According to the Patents Act 1970, there are two types of patents i.e. product patents and process patents. The product patent is the end result or the output produces of a product and the process patent is the journey of a patent being produced. Under the Patent Act, both processes and products are entitled to qualify as inventions if they are new, involve an inventive step, and are capable of industrial application.
For e.g. Paracetamol Tablets can be considered as a product patent while the manufacture of the tablets is the process patent. In the patents, there is no transfer of rights and nor is the concept of Moral or Economic rights. The prime example being that the medicines or the vaccines created by the first country for the COVID-19 will have to share it with the other countries as well.
Under section 2(1)(j) of the Act, ‘invention’ is described as a new product or method requiring an inventive phase capable of industrial usage. The word ‘industrial application’ applies to an invention capable of industrial application which implies that the invention is capable of being produced or used in the industry. Some of the preconditions of the invention are that it would be new, i.e. the technology submitted for patenting was not in the public domain or is not part of the state of the art. The term Patent is defined under Section 2(m) of the Act.
Who is a Patentee?
Patentee is an individual who is registered in the patent registry as the grantee or patent proprietor for the time being. The patentee shall have the right to deal with his property in the same manner as the owner of any other movable property dealing with his property.
Rights of Patentee
Section 48 of the Act, talks about the rights granted to a patentee.
- A patentee has the exclusive right to make use, exercise, sell or distribute the patented article or substance in India, or to use or exercise the method or process if the patent is for a person. This right can be exercised either by the patentee himself or by his agent or licensees. The patentee’s rights are exercisable only during the term of the patent.
- A patentee has the discretion to transfer rights or grant licenses or enter into some other arrangement for a consideration. A license or an assignment must be in writing and registered with the Controller of Patents, for it to be legitimate and valid. The document assigning a patent is not admitted as evidence of title of any person to a patent unless registered and this is applicable to assignee not to the assignor.
- A patentee has the right to surrender his patent, but before accepting the offer of surrender, a notice of surrender is given to persons whose name is entered in the register as having an interest in the patent and their objections, if any, considered. The application for surrender is also published in the Official Gazette to enable interested persons to oppose.
- A patentee has a right to institute proceedings for infringement of the patent in a District Court having jurisdiction to try the suit.
Requirements to Qualify as Invention
- The invention must be new i.e. the invention must be novel, meaning that the Invention must not be in existence;
- The invention must be non-obvious i.e. the invention must be a significant improvement to the previous one; a mere change in technology will not give the right of the patent to the inventor;
- The invention must be useful in a bonafide manner i.e. invention must not be solely used in any illegal work and is useful to the world in a bonafide manner.
- An Invention must involve an inventive step;
- The invention must be capable of industrial application or utility;
- The invention shouldn’t come under the inventions which are not patentable as defined under Section 3 and 4 of the Patent Act of 1970.
Under Section 2(l) of the act, the Novelty or the new invention or the new product is any invention or the technology and is not anticipated by the prior publication in or outside India. The novelty shall not fall in public domain or form part of any prior act. Patents can be globally registered. The patent is always given for new inventions. It shouldn’t have been invented in our country or outside of our country so that it becomes a novelty. If a pen is invented, and it has not been anticipated anywhere else, then it’d be considered a novelty. The patent is entitled to the one who files the application first and not to the one who has invented it first. Section 3 is the exception to the patents and needs to be learned thoroughly and everything in the world is patentable except few.
What is Prior art?
Existing knowledge in the field of claimed invention or knowledge is called the Prior Art. To satisfy the criteria of novelty, it should be: –
- Any invention or technology.
- It should not have been anticipated.
- It should not be a prior art or an art of the public domain.
Term of Patent
In India, the duration of each patent is 20 years from the date of filing the patent application, irrespective of whether it is filed with provisional or full specification. However, in the case of requests submitted under the Patent Cooperative Treaty (PCT), the 20-year period begins from the international filing day.
Patent can be qualified only if all three criteria are fulfilled respectively i.e. of Novelty, Inventive Step and being Capable of Industrial application, failure of any of the three will result in the patent as unqualified and thus, the application for the same will be rejected.
What cannot be patented?
Section 3 deals with the exceptions of which subject matters are excluded from the patents.
a) Frivolous Inventions: These are contrary to natural laws. E.g. If any invention runs from the blood of a human and nothing else. This would be contrary to the natural laws and will come under the frivolous inventions. Something always against the establishment of natural laws.
b) Inventions against public order or morality or which causes harm to the environment.
c) Mere discovery of scientific principles or any discovery. E.G Newton’s theories and other principles etc. If these theories will be patentable, how wills students study them if they are not given freely in the public domain.
d) Mere discovery of a known substance is not patentable.
The NOVARTIS Case
Novartis AG vs. Union of India
In 1998, one of the largest international pharmaceutical companies i.e. Novartis International AG filed an application as per the TRIPS agreement before the Chennai Indian patent office for the grant of a patent for an anticancer drug ‘Glivec’ which is used to treat Chronic Myeloid Leukemia (CML) and Gastrointestinal Stromal Tumours (GIST) invented from Beta crystalline form of “Imatinib mesylate”. This drug is famously used in the treatment of cancer and the same is patented in more than 35 countries.
The issues raised in this case are as follows: –
- According to provision 3(d) of the Patents Act, 1970, what is the meaning of a known substance?
- According to provision 3(d) of the Patents Act, 1970, what is efficacy?
- Whether an increase in bioavailability qualify as an increase in therapeutic efficacy?
- Whether the invention “Beta crystalline form of imatinib mesylate” claimed by Novartis is more efficacious than the substance that it was derived from i.e. “Imatinib mesylate”?
Judgment
In April 2013, the two-judge bench of Supreme Court of India rejected the appeal filed by Novartis and upheld that the beta crystalline form of Imatinib Mesylate is a new form of the known substance i.e., Imatinib Mesylate, wherein the efficacy was well known. Supreme Court made it crystal clear that in the case of medicine “Efficacy” in section-3(d) only means “Therapeutic Efficacy” and states that all properties of a drug are not relevant, the properties which directly relate to efficacy in the case of medicine are its therapeutic efficacy. Supreme Court in the third issue ruled that about 30% increase in bioavailability qualifies as an increase in therapeutic efficacy under section-3(d) of Patent Act, 1970 if the evidence is provided for the same. Supreme Court compared the efficacy of “Beta Crystalline form of Imatinib Mesylate” with “Imatinib Mesylate” with reference to its flow properties, better thermodynamic stability, and lower hygroscopicity, and held that none of these properties contribute to increasing in therapeutic efficacy according to section-3(d) of Patent Act, 1970 and Novartis not provided any document that shows that the efficacy of “Beta Crystalline form of Imatinib Mesylate” is more as compared to the efficacy of “Imatinib Mesylate”.
e) Mere admixing of mixtures which can lead to aggregation in properties are non-patentable. This exception is generally for Chemical substances such as H20, H2So4, etc. The mere addition of elements does not form a completely new product and thus is not patentable. The addition shall give rise to the synergistic effect i.e. the amalgamation shall have a greater effect than the separate effects.
f) Mere aggregation or duplication of devices in a known way so that they work independently is not an invention. The mere addition of different substances and any combination of different matters is not patentable. For e.g. a torch added in the pen for its better functioning.
g) Repealed
h) Horticulture or Agricultural methods is not patentable. For e.g. the Mere cultivation of algae or the mushrooms is not patentable.
i) Medicinal, curative, prophylactic, diagnostic, therapeutic for treating diseases in humans and animals are non-patentable. Medicinal methods, curative methods, or surgical methods for the cure of diseases are not patentable.
j) The essential biological process for the well-being of plants and animals is not patentable.
k) Simple mathematical business or computer program.
l) Any aesthetic creation which comes under Copyrights is not patentable.
m) Any mental act or rule or method of playing a mental game. E.G. of such games is Ludo, Chess, Sudoku, etc.
n) Any presentation such as railway timetables or the calendar is not patentable.
o) Any topography of integrated circuits is not patentable
p) Traditional Knowledge is not an invention. Any traditional knowledge such as antiseptic properties of turmeric is not patentable.
Section 4 talks about the inventions relating to atomic energy and they are not patentable. It deals with inventions relating to atomic energy, that are also not patentable and that fall within subsection (1) of section 20 of the Atomic Energy Act, 1962.
Patent filing application
The patent can be filed by the two ways namely; provisional specification and complete specification. The patent will be published right after 18 months from the date of filing. If the person wants to get the patent published before 18 months, he will have to pay the statutory fee for the publication. A requisite form can be filled or a fee can be paid for the same. The rule of jurisdiction has to be followed and the application will be filed accordingly, as per the jurisdiction. The second step for publication is that the request for examination shall be in 48 months. The third step is the examination and it’s not automatic unlike the publication and the applicant will have to fill the requisite form for the publication. Anyone can file it on behalf of the applicant. Timeline is 48 months for filing of the application from the priority date. The patent examiner will be according to the patent orientation. The patent examiner will check the patent database and other credentials on the three important criteria for the patent. The examiner will check the applicability under sections 3 and 4 respectively. The examiner will check the clerical mistakes as well. The drawing sheets and formatting guidelines are to be followed as well. The first examination report will be created after the patent is being ensured as authentic by the examiner. A reply shall be given within the 6 months of the first examination report by the applicant. After the reply given for the examination, if the examiner needs further clarification for the invention, he shall have the applicant present physically or via video conference.
A parallel system runs in the patent application for the opposition of the patent. There can be two types of opposition, namely, the pre-grant opposition and the post-grant opposition. The pre-grant opposition is filed after the publication and before the grant of the patent and it can be filed by anyone, whereas the post-grant opposition can be filed only by the person skilled in the art. The oppositions shall be substantiated by the evidence and the grounds must be followed before the filing of the opposition. The timeline for filing post-grant opposition is one year after the grant of patent and not after that.
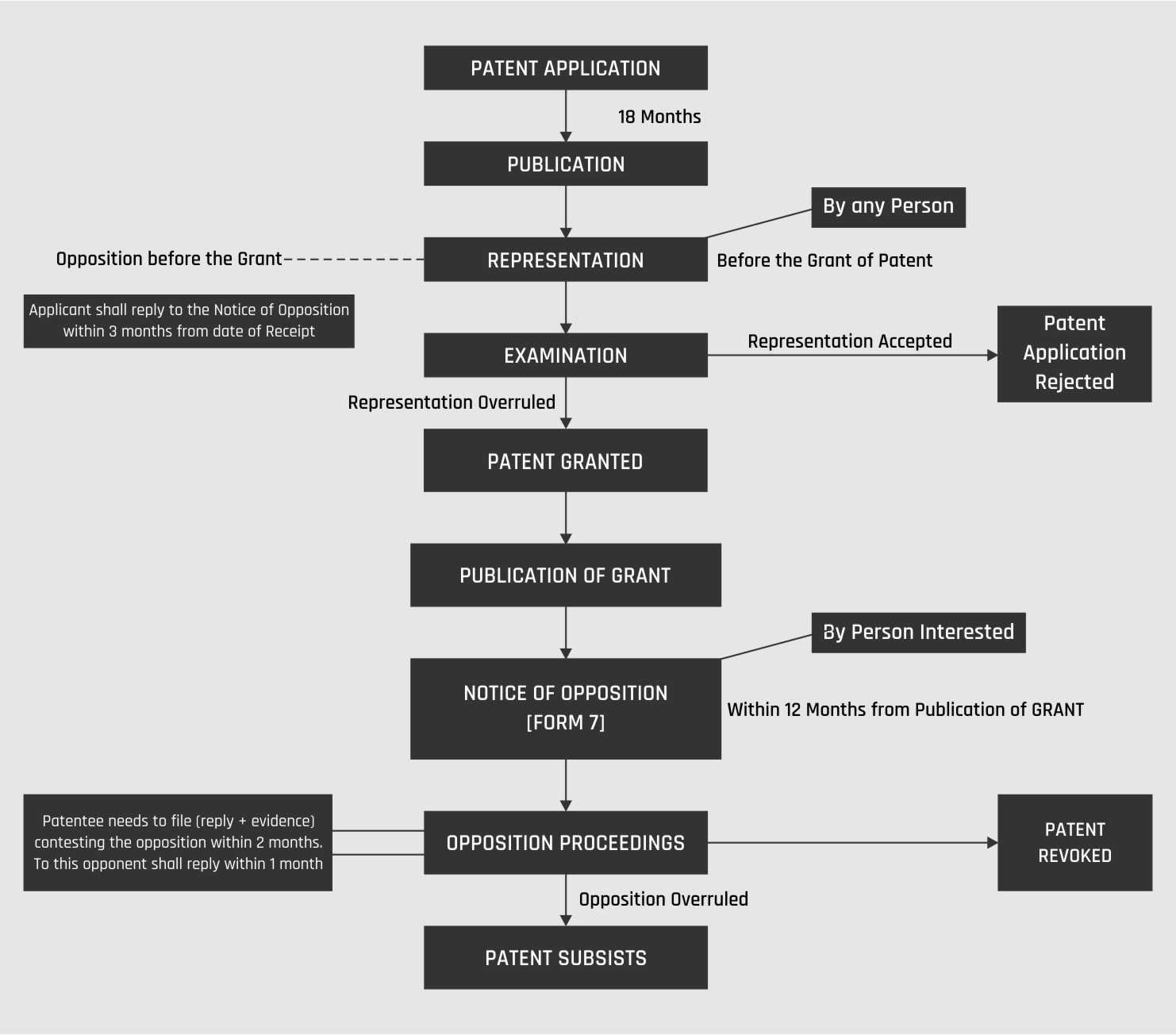
The sections associated with the patent application are: –
Sections 6 and 7 specifically related to the application. The application can be filed by three persons namely the True and first inventor; the assignee of the first inventor; and the legal representative of the deceased person who was about to make the application.
Section 9 and 10 deals with the provisional and complete specifications respectively.
Section 11 deals with the priority date. If a person is directly filing under the complete specification, then the priority date will be the filing of the complete specification, whereas if the person has filed a provisional specification and then the complete specification after 12 months of the former, then the priority date will be the date of filing of the provisional specification.
Section 11A talks about the publication. From the date of publication, the patent is being protected. Rights such as rights of infringement and authority ownership are given. Section 11B talks about the examination of the patent.
Section 25 deals with the opposition proceedings namely pre-grant and post-grant opposition.
Section 43 talks about the grant of patent.
Section 45 talks about the date of the patent. Date of the patent will be the priority date only, but the date of the patent is the filing of the application for calculating the term of 20 years of the patent.
Compulsory License
Compulsory Licensing is one of the most important aspects of the Indian Patents Act 1970, subject to certain conditions being fulfilled. At any period after the expiry of three years from the date of the sealing of a patent, any interested party may, subject to the following conditions, apply to the Patent Controller for the issuance of a compulsory patent license. If a person grants permission to use any product, then it would be called a voluntary license. The first stage is taking a voluntary license. There are three grounds on which you can ask for the compulsory license. The license can be asked for any time after 3 years of the grant of the patent. Any person can ask for a compulsory license. Be it a natural person, artificial person, or a company.
Grounds for granting of compulsory license
- The reasonable demand shall be there.
- The product shall not be reasonably in public domain.
- The patented invention is not worked in the territory of India.
If any country makes the vaccine for the COVID 19, the other countries shall be given the vaccine for the same. Public health is the agenda for the WHO and the TRIPS. The Government has released a norm that the process of the vaccine shall be granted as a compulsory license for the other countries. PPP is the triangle of public health, patent, and pandemic and it is a relative issue in this crisis.
Section 88 talks about the powers of the controller regarding the compulsory license. In considering the application, the nature of the invention shall be seen by the controller and whether the invention has the ability or not.
Infringement of patents
Patent infringement is a crime concerning the unlawful use, produce, sell, or offer or sell of the subject matter or proprietary invention by another. There are several various patent forms of utility patents, design patents, and plant patents. The fundamental principle underlying patent infringement is that unauthorized persons are not able to use inventions without the consent of the proprietor. Patent infringement occurs directly or indirectly.
Direct patent infringement: The most common form of infringement is direct infringement, where the Invention that infringes patent claims is actually described, or the Invention performs substantially the same function.
Indirect patent infringement: It is divided into two types: –
- Infringement by inducement is any activity by any third party that causes another person to infringe the patent directly. This may include selling parts that can only be used realistically for a patented invention, selling an invention with instructions to use in a certain method that infringes on a method patent or licenses an invention that is covered by the patent of another. The inducer must assist intentional infringement, but does not require intent to infringe on the patent.
- Contributory infringement is the sale of components of material that are made for use in a patented invention and have no other commercial use. There is a significant overlap with indications, but contributor violations require a high level of delay. Violations of the seller must have direct infringement intent. To be an obligation for indirect violations, a direct violation must also be an indirect act.
Remedies
- Monetary Relief: It is a form of compensatory damages are available to prevent patent infringement.
- Equitable relief: Orders are issued by the court to prevent a person from doing anything or Act. They might be in two forms – preliminary injunction which are orders made in the initial stage of lawsuits or lawsuits that prevent parties from doing an act that is in dispute (such as making a patent product). A permanent injunction is a final order of a court that permanently ceases certain activities or takes various other actions.
Case Laws
Hoffman vs. Cipla
Case filed by Hoffman against Cipla ltd and an enhanced version was produced by Cipla Ltd. Product vs Substance dichotomy case. Meaning of Substance? When a substance becomes a product and how it becomes patentable. Substance and enhanced efficacy lead to a new product and novelty come up. Substance per se not patentable but a new product with enhanced efficacy is patentable. This case is also called the la Hoffman case.
Diamond vs Chakraborty
This case is on micro-organisms. Genetically engineered bacteria. Process patent was demanded over. A patent was claimed over the process, product, and components.
First issue: -Bacteria is a living thing, then can it be patentable. SC said that human-made organisms are patentable and the second thing stated was that subject matter to include anything made by man is patentable. Only micro-organisms in living beings are patentable.
Windsurfing International Inc. vs Tabur Marine.
Pozzoli test
To identify person skilled in the art and then identify the inventive concept which cannot be readily construed. Are these differences any obvious differences or not. It is a four-step test to test the inventive step.
Dhanpat Seth vs. Union of India
Dhanpath produced Kilta alike device using bamboo, which can be used for manual agriculture and it was light in weight and they started to produce and manufacturing and Neel Kamal plastic started using that without paying the royalty to Dhanpath and it forms the traditional knowledge. Dhanpath said that the original Kilta was painful and heavy to use and for this reason only, they started using the basket. Whether valid patent lies with Dhanpath Seth?
The second issue in the case was Injunction and the third was Infringement.
Court held that bamboo baskets don’t complete the novelty step and the traditional knowledge clause. It was a mere duplication of Kilta and the usefulness was not shown and there is no inventive step and it reiterates the property.
(This article has been written and submitted by Mr. Prakhar Agnihotri during his course of internship at B&B Associates LLP. Mr. Agnihotri is a fourth year law-student at University of Petroleum & Energy Studies, Dehradun, Uttrakhand.)